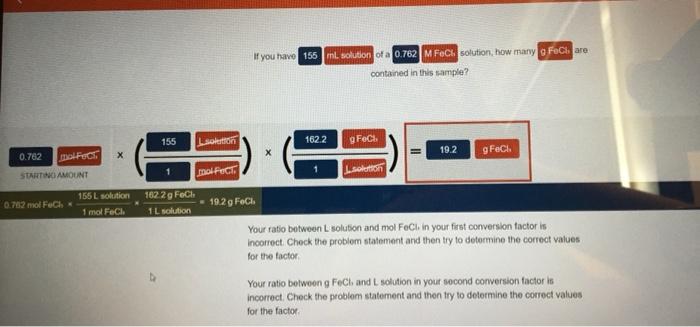
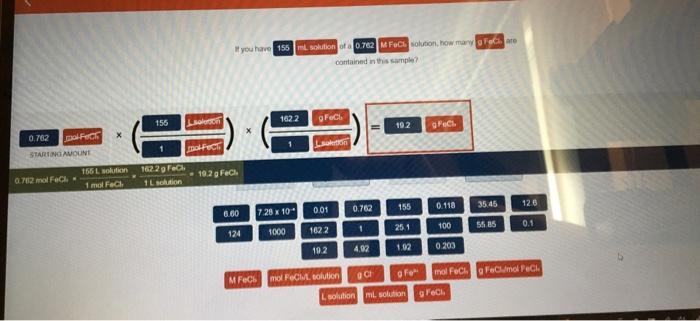
Embark on a journey into the realm of solution concentration, where we unravel the intricacies of if you have 155 ml solution of a 0.762. Join us as we delve into the captivating world of molarity, volume, and their profound impact on the world of chemistry.
In this comprehensive guide, we will illuminate the fundamental concepts of solution concentration, empowering you to master the art of calculating molarity and volume. Prepare to unlock the secrets of solution chemistry and gain invaluable insights into this fascinating field.
Solution Concentration: If You Have 155 Ml Solution Of A 0.762
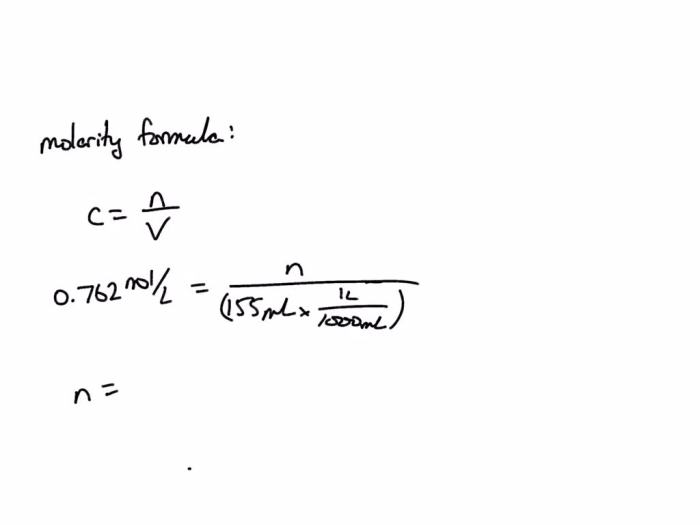
Solution concentration refers to the amount of solute dissolved in a given amount of solvent. It is a crucial aspect of chemistry as it determines the properties and behavior of solutions. To calculate the concentration of a solution, we use the following equation:
Concentration = Amount of Solute / Volume of Solution
where concentration is expressed in units of molarity (M), amount of solute in moles, and volume of solution in liters.
Solution Volume
Solution volume plays a significant role in determining the amount of solute present. The volume of a solution is directly proportional to the amount of solute dissolved in it. To calculate the volume of a solution, we use the following equation:
Volume of Solution = Amount of Solute / Concentration
where volume of solution is expressed in liters, amount of solute in moles, and concentration in molarity (M).
Molarity and Volume Calculations

To calculate the molarity of a solution, we divide the amount of solute (in moles) by the volume of the solution (in liters). To calculate the volume of a solution, we divide the amount of solute (in moles) by the molarity of the solution (in M).
| Calculation | Equation |
|---|---|
| Molarity | Molarity = Amount of Solute (moles) / Volume of Solution (liters) |
| Volume | Volume of Solution (liters) = Amount of Solute (moles) / Molarity |
Solution Dilution and Concentration
Solution dilution refers to the process of adding more solvent to a solution, which decreases its concentration. The dilution equation is used to calculate the concentration of a diluted solution:
C1V1 = C2V2
where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
FAQ Corner
What is the significance of solution concentration?
Solution concentration plays a crucial role in determining the properties and behavior of solutions. It influences factors such as reactivity, solubility, and colligative properties, providing valuable insights into the nature of chemical systems.
How do I calculate the molarity of a solution?
To calculate the molarity of a solution, simply divide the number of moles of solute by the volume of the solution in liters. The formula is: Molarity (M) = Moles of Solute / Volume of Solution (in liters)
What is the relationship between solution volume and concentration?
Solution volume and concentration are inversely related. As the volume of a solution increases, the concentration decreases, and vice versa. This relationship is crucial for understanding the effects of dilution and concentration on solution properties.