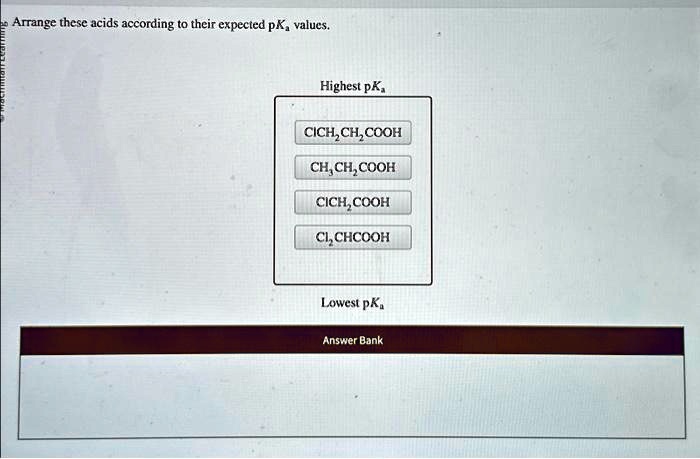
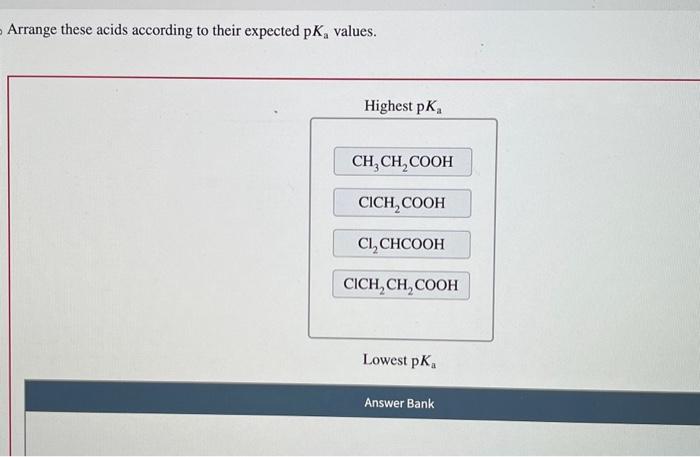
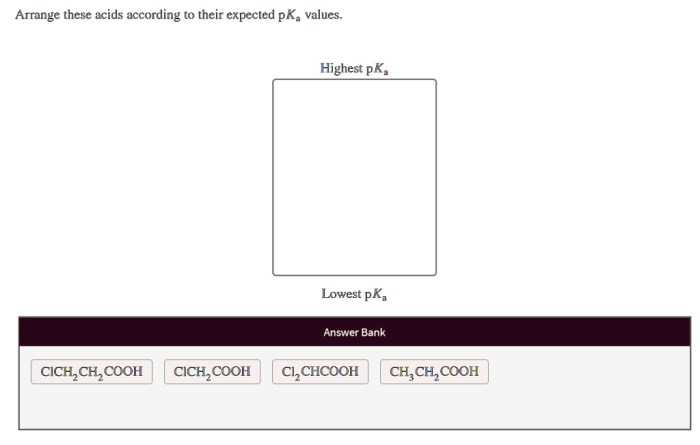
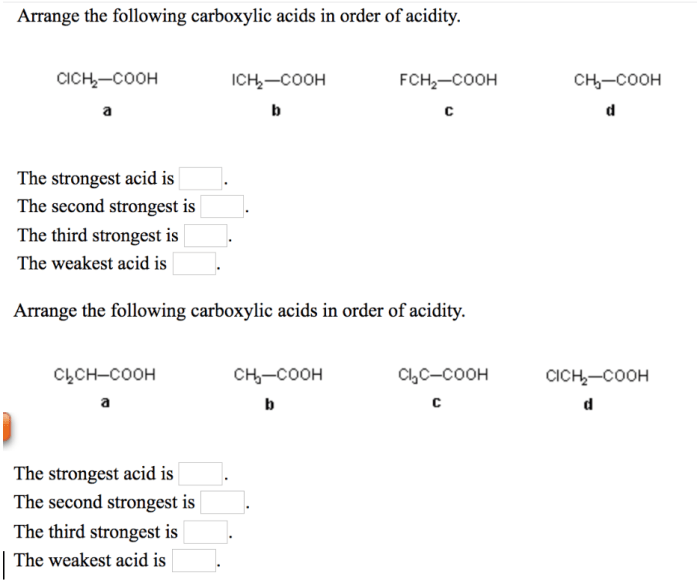In the realm of chemistry, understanding the properties of acids is crucial. Arrange these acids according to their expected pKa values, delves into the fascinating world of acid strength, exploring the factors that govern their behavior and their diverse applications across various scientific disciplines.
This comprehensive guide unravels the intricate relationship between acid structure and pKa values, providing a deeper understanding of how molecular architecture influences acid strength. By examining a range of acids, from simple carboxylic acids to complex phenols, we uncover the subtle nuances that dictate their relative acidity.
Acid Properties: Arrange These Acids According To Their Expected Pka Values
Acids are substances that can donate protons (H+ ions). The strength of an acid is measured by its pKa value, which is the negative logarithm of its acid dissociation constant (Ka). The lower the pKa value, the stronger the acid.
Several factors influence pKa values, including electronegativity, hybridization, and resonance. Electronegativity is the ability of an atom to attract electrons towards itself. The more electronegative the atom bonded to the acidic proton, the stronger the acid. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals.
The more s-character in the hybrid orbital containing the acidic proton, the stronger the acid. Resonance is the delocalization of electrons over multiple atoms or bonds. Resonance can stabilize the conjugate base of an acid, making the acid weaker.
Acid Structures
Acids can have different structures, including carboxylic acids, alcohols, and phenols. Carboxylic acids have the general structure RCOOH, where R is an alkyl or aryl group. Alcohols have the general structure ROH, where R is an alkyl or aryl group.
Phenols have the general structure ArOH, where Ar is an aryl group.
The structural features of an acid can affect its pKa value. For example, carboxylic acids are generally stronger acids than alcohols, which are in turn stronger acids than phenols. This is because the electronegative oxygen atom in carboxylic acids attracts electrons away from the acidic proton, making it more likely to dissociate.
The oxygen atom in alcohols is less electronegative than in carboxylic acids, so it attracts electrons less strongly, making the acidic proton less likely to dissociate. The oxygen atom in phenols is even less electronegative than in alcohols, so it attracts electrons even less strongly, making the acidic proton even less likely to dissociate.
Acid Strength Comparison
The following table lists some common acids along with their structures, names, and expected pKa values:
| Structure | Name | pKa |
|---|---|---|
| CH3COOH | Acetic acid | 4.76 |
| CH3CH2OH | Ethanol | 15.9 |
| C6H5OH | Phenol | 10.0 |
As can be seen from the table, acetic acid is the strongest acid, followed by ethanol, and then phenol. This is consistent with the trends in pKa values discussed above.
Acid Applications, Arrange these acids according to their expected pka values
Acids have a wide range of applications in chemistry, biology, and medicine. In chemistry, acids are used as catalysts for a variety of reactions. In biology, acids are involved in many metabolic processes, such as the digestion of food and the production of energy.
In medicine, acids are used to treat a variety of conditions, such as heartburn and indigestion.
The pKa value of an acid is an important factor in determining its applications. For example, strong acids, such as hydrochloric acid, are used to dissolve metals and to etch glass. Weak acids, such as acetic acid, are used as food additives and as preservatives.
The pKa value of an acid also determines its suitability for use in biological systems. For example, acids with pKa values close to 7 are often used as buffers to maintain a constant pH.
FAQs
What is the significance of pKa values in acid chemistry?
pKa values quantify the strength of acids, indicating their tendency to donate protons. Lower pKa values correspond to stronger acids that readily release protons, while higher pKa values indicate weaker acids that hold onto protons more tightly.
How do structural features influence pKa values?
Structural features, such as electronegativity, hybridization, and resonance, can significantly affect pKa values. Electron-withdrawing groups, like halogens, lower pKa values by stabilizing the conjugate base, making the acid stronger. Conversely, electron-donating groups, like alkyl groups, raise pKa values by destabilizing the conjugate base, weakening the acid.
What are some practical applications of acids with different pKa values?
Acids with varying pKa values find applications in diverse fields. Strong acids, like hydrochloric acid, are used in industrial processes and as laboratory reagents. Weak acids, like acetic acid, are employed in food preservation and as solvents. The controlled release of protons from acids with specific pKa values is also crucial in drug delivery systems and pH regulation in biological systems.