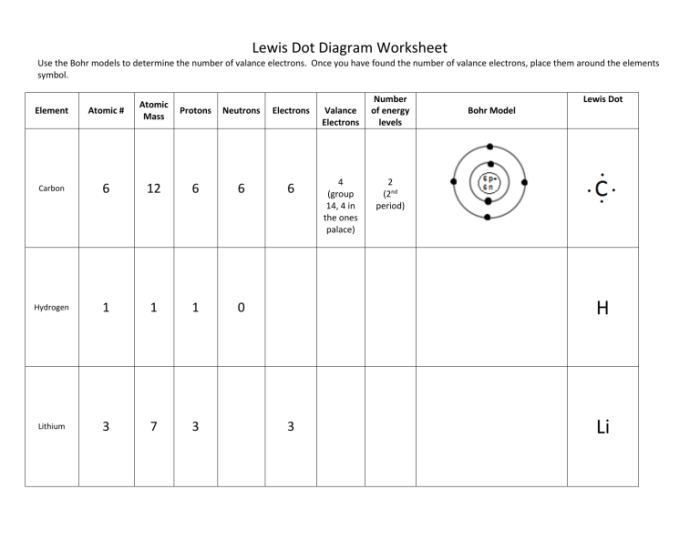
Bohr and Lewis dot diagrams, fundamental tools in chemistry, unveil the intricate world of atoms and molecules. These diagrams, developed by Niels Bohr and Gilbert N. Lewis, provide a visual representation of the arrangement of electrons in an atom or molecule, offering invaluable insights into their chemical properties and behavior.
By delving into the history, applications, and limitations of Bohr and Lewis dot diagrams, we embark on a captivating journey that explores their pivotal role in predicting chemical properties, explaining chemical bonding, and extending our understanding of molecular structures. These diagrams serve as a cornerstone for various scientific disciplines, empowering us to design new materials, advance computational chemistry, and delve into the realm of nanotechnology.
Bohr and Lewis Dot Diagrams
Bohr and Lewis dot diagrams are two complementary models that represent the structure of atoms and molecules. They provide a simplified visualization of the arrangement of electrons within an atom or molecule, helping us understand their chemical properties and behavior.
Historical Context
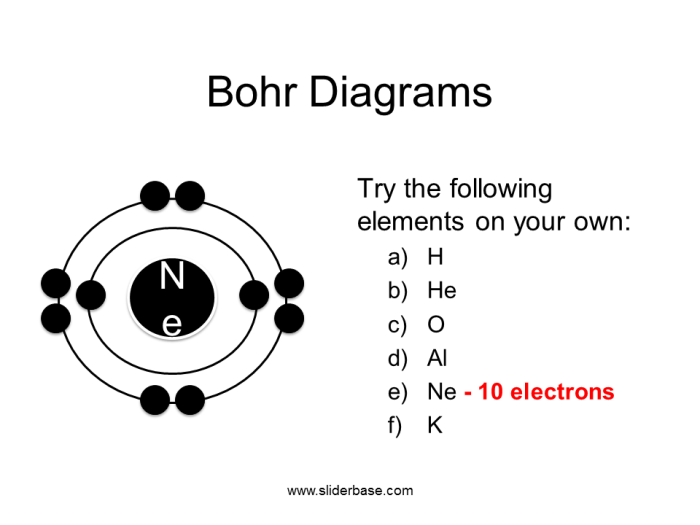
The Bohr model was developed by Niels Bohr in 1913, based on his theory of the hydrogen atom. It depicts electrons orbiting the nucleus in discrete energy levels, with each level corresponding to a specific energy value. Later, in 1916, Gilbert N.
Lewis proposed the Lewis dot diagram, which represents the valence electrons of an atom as dots surrounding the atomic symbol.
Limitations
While Bohr and Lewis dot diagrams are useful for understanding basic atomic and molecular structures, they have certain limitations. The Bohr model does not account for the wave-particle duality of electrons and the uncertainty principle. Additionally, Lewis dot diagrams do not provide information about the three-dimensional shape of molecules or the bonding between atoms.
Applications of Bohr and Lewis Dot Diagrams
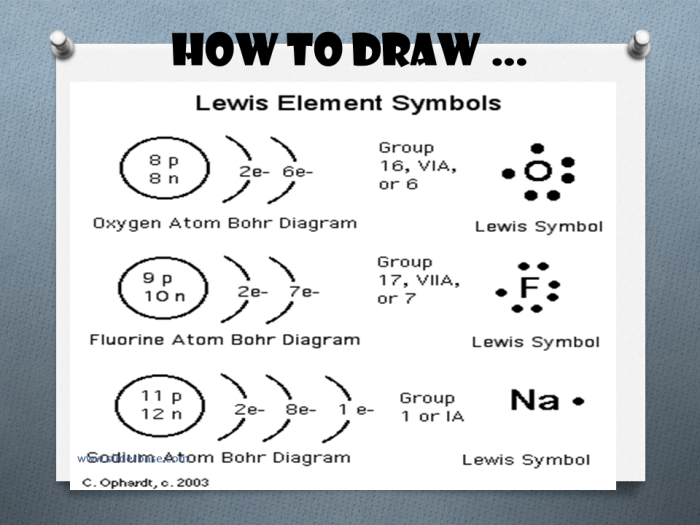
Bohr and Lewis dot diagrams are powerful tools for understanding and predicting the chemical properties of elements and compounds. They provide a visual representation of the electronic structure of atoms and molecules, which can be used to explain chemical bonding and reactivity.
Predicting Chemical Properties of Elements
The arrangement of electrons in an atom’s Bohr diagram determines its chemical properties. Elements with similar electron configurations tend to have similar chemical properties. For example, all alkali metals have one valence electron in their outermost shell, which makes them highly reactive and prone to forming ionic bonds.
Explaining Chemical Bonding
Lewis dot diagrams show the valence electrons of atoms and how they interact with each other to form chemical bonds. Covalent bonds form when two atoms share electrons, while ionic bonds form when one atom transfers electrons to another. The number and arrangement of valence electrons in the Lewis dot diagram can predict the type of bond that will form.
Applications in Various Fields of Science, Bohr and lewis dot diagrams
Bohr and Lewis dot diagrams are used in various fields of science, including:
- Chemistry:To understand chemical bonding, predict chemical properties, and design new materials.
- Biology:To study the structure and function of biological molecules, such as proteins and DNA.
- Materials Science:To develop new materials with specific properties, such as semiconductors and superconductors.
- Education:To teach students about the fundamental principles of chemistry and atomic structure.
Extensions of Bohr and Lewis Dot Diagrams
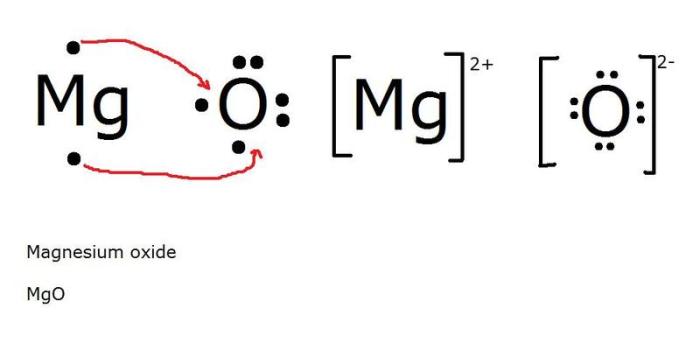
The Bohr model and Lewis dot diagrams are useful tools for understanding the electronic structure of atoms and simple molecules. However, they have limitations and cannot fully explain the behavior of more complex molecules.
Molecular Orbital Theory
Molecular orbital theory (MO theory) is an extension of the Bohr model and Lewis dot diagrams that provides a more accurate description of the electronic structure of molecules. MO theory describes the electrons in a molecule as occupying molecular orbitals, which are regions of space around the nuclei where the electrons are most likely to be found.
Molecular orbitals are formed by the combination of atomic orbitals. The number and type of molecular orbitals that are formed depend on the number and type of atomic orbitals that are available.
MO theory can be used to explain a wide range of molecular properties, including bond lengths, bond strengths, and chemical reactivity.
Example
The following is an example of how MO theory can be used to explain the electronic structure of a molecule:
The hydrogen molecule (H 2) is formed by the combination of two hydrogen atoms. Each hydrogen atom has one electron in its 1s atomic orbital.
When the two hydrogen atoms come together to form a molecule, their 1s atomic orbitals overlap to form a molecular orbital. This molecular orbital is called the σ 1smolecular orbital.
Understanding Bohr and Lewis dot diagrams can help visualize electron configurations and chemical bonding. If you’re looking for further insights, check out the Bill Nye Genes Answer Key for an engaging and informative resource. Returning to Bohr and Lewis dot diagrams, these tools provide a fundamental basis for comprehending the behavior of atoms and molecules.
The σ 1smolecular orbital is a bonding orbital, which means that it helps to hold the two hydrogen atoms together. The two electrons in the σ 1smolecular orbital are shared by the two hydrogen atoms.
Advanced Applications of Bohr and Lewis Dot Diagrams
Bohr and Lewis dot diagrams, once confined to understanding atomic structure, have evolved into indispensable tools for designing and analyzing materials at the atomic level. These diagrams provide a powerful framework for predicting and interpreting chemical properties, enabling scientists to push the boundaries of materials science.
Use in Materials Design
Bohr and Lewis dot diagrams serve as a roadmap for designing new materials with tailored properties. By manipulating the electronic configurations of atoms, scientists can engineer materials with specific electrical, optical, and magnetic characteristics. For instance, in the development of semiconductors, Bohr diagrams guide the selection of elements with appropriate energy levels to create materials with desired bandgaps.
Computational Chemistry
Bohr and Lewis dot diagrams are also essential in computational chemistry, where they provide a simplified representation of atomic interactions. By incorporating these diagrams into computational models, scientists can simulate and predict the behavior of molecules and materials. This capability enables the design of new drugs, the optimization of chemical processes, and the understanding of complex chemical reactions.
Nanotechnology
In the realm of nanotechnology, Bohr and Lewis dot diagrams play a crucial role in understanding the electronic properties of nanomaterials. These diagrams help researchers visualize and analyze the quantum confinement effects that govern the behavior of atoms and molecules at the nanoscale.
By tailoring the electronic configurations of nanomaterials, scientists can create materials with unique properties, such as enhanced optical absorption or improved electrical conductivity.
User Queries
What is the difference between a Bohr diagram and a Lewis dot diagram?
Bohr diagrams depict the arrangement of electrons in specific energy levels around the nucleus, while Lewis dot diagrams focus on the valence electrons involved in chemical bonding.
How can Bohr and Lewis dot diagrams predict chemical properties?
By examining the number and arrangement of valence electrons, these diagrams provide insights into an element’s reactivity, electronegativity, and bonding preferences.
What are the limitations of Bohr and Lewis dot diagrams?
These diagrams do not account for the wave-particle duality of electrons and cannot accurately represent the bonding in complex molecules or transition metal complexes.